In its 2007 report on Toxicity Testing in the 21st Century: A Vision and a Strategy, the US National Research Council (NRC) highlighted need for more rapid toxicity testing strategies in order to expand the coverage of the large number of chemicals for which toxicological data is lacking.
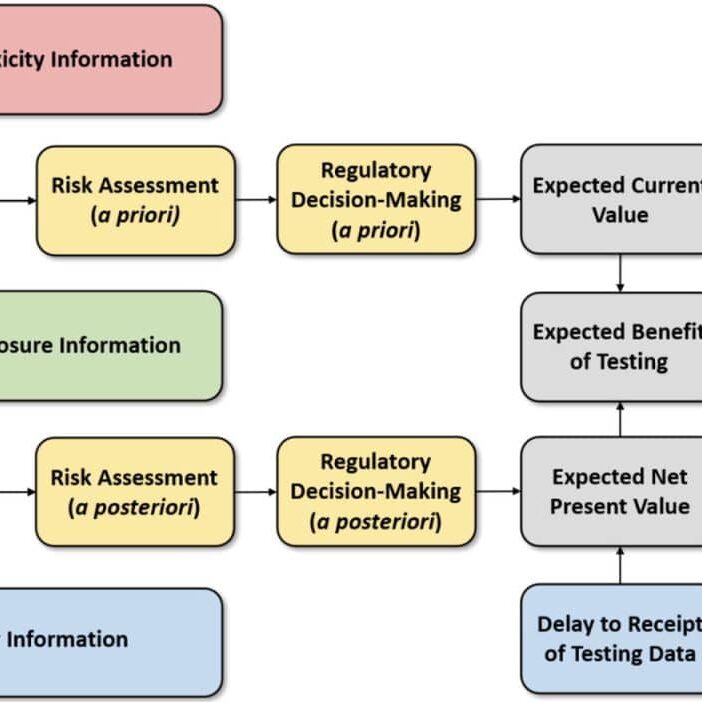
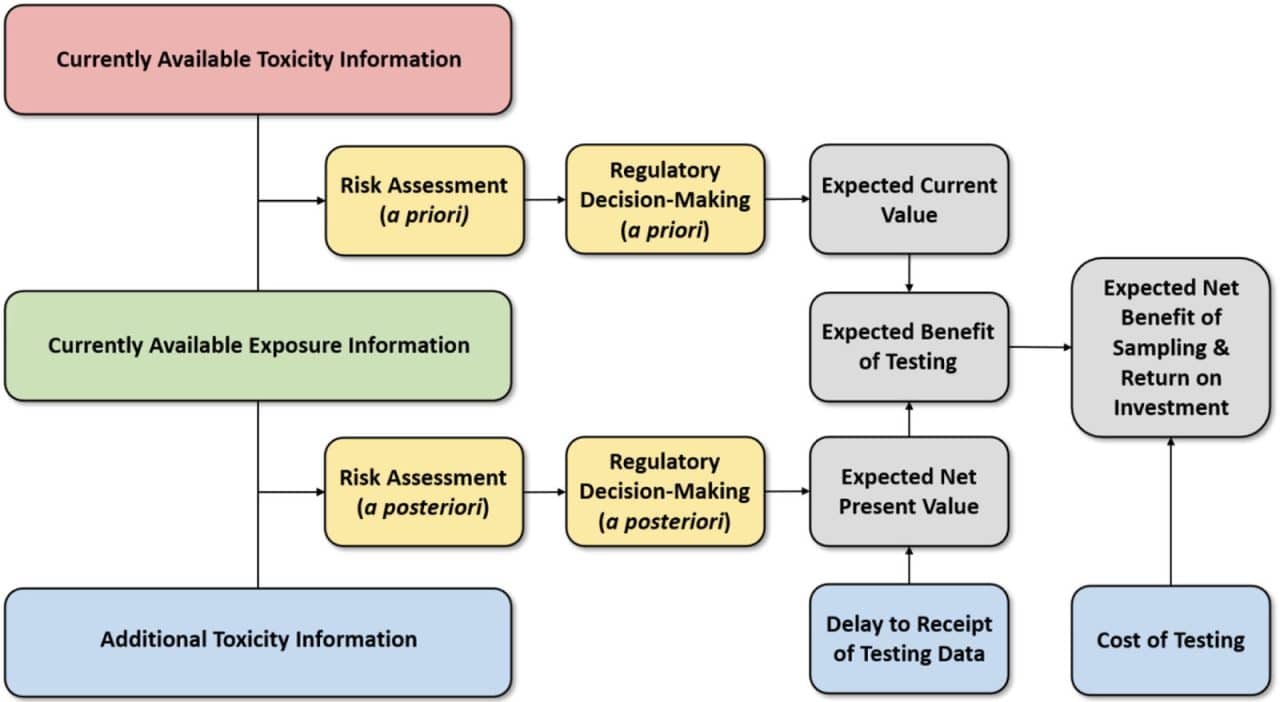
This need was further underscored by the NRC in its 2009 report Science and Decision: Advancing Risk Assessment, which recommended the use of formal value-of-information (VOI) techniques to evaluate alternative testing strategies. Hagiwara et al. (2022) recently developed VOI methods to evaluate the trade-offs between the cost, timeliness, and uncertainty reduction in toxicity testing.
These contributions suggest that while rapid alternative test methods may be subject to greater uncertainty than traditional more expensive animal tests of longer duration, having sufficient information to support risk decision making in a timely manner can result in a lower total social cost by avoiding pubic health impacts that can accrue when decisions are delayed pending the results of toxicity tests of longer duration. Although further VOI analyses are needed to reaffirm these findings under broader real-world toxicity testing scenarios, initial results appear to be supportive of alternative test methods that can provide cost-effective toxicity data in a timely manner.
Posted in RSI News
More RSI News
Climate change modelling for the Bow River watershed
Developing a suite of watershed-scale climate models.
Read News ItemQuarterWatch analyzes MedWatch Reports
This issue of QuarterWatch analyzes MedWatch Reports from the third quarter of 2015. This issue identifies major differences in reports of cancer associated with drugs…
Read News Item