Client: Health Canada
Listing of the client in no way affirms the client's support, sponsorship, or validation in any form of Risk Sciences International or the RSI staff member(s) who conducted this project during their stay with RSI or prior to joining the company. This case study is displayed for informative purposes only to demonstrate the capacity of RSI staff members. This case study reveals no proprietary information or information deemed sensitive.
Contraceptive recall review
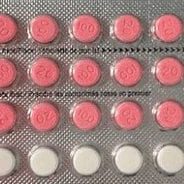
Health Canada requested an external review following significant public criticism of its handling of a pharmaceutical recall. The case involved a contraceptive drug that was withdrawn due to packaging errors, and the department faced questions about whether it had acted swiftly and transparently enough. The client sought an impartial evaluation to understand the sequence of events, the adequacy of internal processes, and the way the recall had been perceived by the public and media.
The evaluation covered multiple layers. RSI reviewed the regulatory and procedural basis for recall decision-making, as outlined in the governing legislation, regulations, and departmental policies. Key officials involved in the recall were interviewed to document how decisions had been made in practice. A detailed chronology of the recall process was assembled, capturing both internal actions and external communications, to allow for a step-by-step evaluation. Finally, media reporting and public reactions were analyzed to highlight how the recall was framed publicly and where concerns were most acute.
Health Canada asked for actionable recommendations to strengthen its recall processes. RSI delivered a set of proposals under four themes: improve timeliness in responding to company notifications; clarify and communicate roles and responsibilities for recalls; integrate social concern into future recall decisions; and update legislation to clarify drug company notification requirements. The overarching purpose was to provide Health Canada with a roadmap for more effective, transparent, and publicly trusted recall management.
Experts related to this case study
More RSI Case Studies
RSI presents a very small selection of case studies to highlight some of its key work.