Case study client: Alliance of Blood Operators
Listing of the client in no way affirms the client's support, sponsorship, or validation in any form of Risk Sciences International or the RSI staff member(s) who conducted this project during their stay with RSI or prior to joining the company. This case study is displayed for informative purposes only to demonstrate the capacity of RSI staff members. This case study reveals no proprietary information or information deemed sensitive.
Mandate
The Alliance of Blood Operators (ABO) needed a coherent risk management framework to guide decision-making on the delivery of blood services by its member organizations, which would help produce consistent decision-making processes and outcomes across the different national jurisdictional and regulatory structures. The RBDM approach was meant to support a shift in risk decision-making in national blood services organizations from one focused on minimizing all risks to blood quality and supply, with little concern for costs, to a risk-based approach in which resources are allocated according to risk and the application of financial resources and management rigour is proportional to the risk they are addressing.
Work
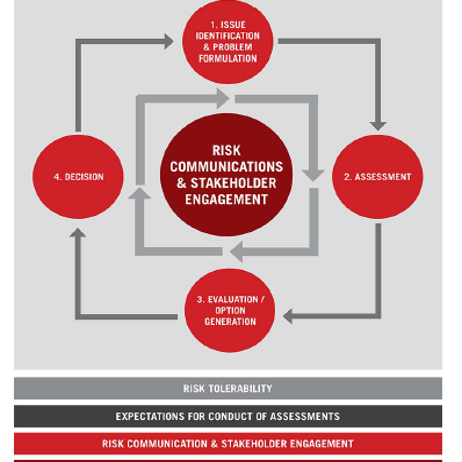
The project proceeded in a series of steps, in collaboration with an international project team of ABO representatives. The first step was a literature review of risk-based regulatory frameworks and the identification of elements that would be appropriate for the ABO framework. The second step was the development of a overall framework consisting of the main elements that were needed; an innovative element of this framework is that a set of foundational policy elements, including risk management principles, transparency policy, communication and stakeholder engagement, were developed, and depicted in a framework diagram as a means of making them explicit and building trust in the blood system.
The major management processes were then designed, with worksheets and decision support tools where these were helpful; these consisted of problem formulation, including clear identification of receptor and vulnerable groups; health risk, health economics and other types of assessments; ethical and risk tolerability evaluations; and a process for systematically evaluating the benefits of each potential decision option. RSI drafted guidelines and worksheets for several of the process steps.
Several workshops were held to test the utility of the draft framework elements, including an online workshop with international team members that worked through a blood decision scenario, and a workshop session at an international blood operators’ conference. Feedback and experience from these workshops were incorporated into subsequent drafts until the final format was approved.
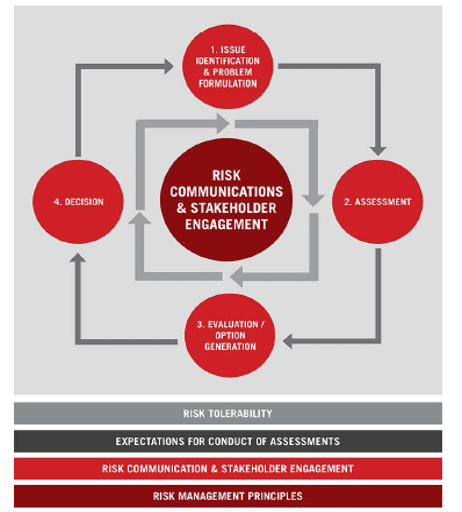
Framework Diagram from RSI submission to Canadian Blood Services, April 2, 2015.
The foundational policy elements – risk management principles, policy for transparency, communication and stakeholder engagement, and risk tolerability - were included in the framework to orient users and the wider community to the ethical and deliberative approach of the decision-making approach.
These elements were shown in the overview graphic to illustrate their function as system-level capacities that support and inform all decision-making.
Outcomes
The ABO RBDM was officially approved and posted on its website for the use of all members. A peer-reviewed article was published (Menitove et al., 2014) that discussed the development of the Framework to support ABO member organizations in the transition to risk-based decision-making.
In 2016, RSI participated in an application of the Framework by Canadian Blood Services to formulate and decide on a strategy for the collection of plasma in Canada. This use of the systematic decision framework was very successful in the CBS developing its plasma strategy.
Also in 2016 RSI was asked for a further elaboration of the risk tolerability process and support tools. These tools were developed and refined, and have been successfully employed in the evaluation of several decisions.
Posted in Case Study
More RSI Case Studies
RSI presents a very small selection of case studies to highlight some of its key work.
The review involved an analysis of the events and processes that led to public criticism of Health Canada’s handling of the drug company’s recall of a contraceptive drug due to…
Read MoreRSI was tasked with providing scientific advice and content for a nuclear power reactor licensing process in an OECD country. The nuclear safety commission in question regulates and licenses all…
Read More- « Previous
- 1
- 2